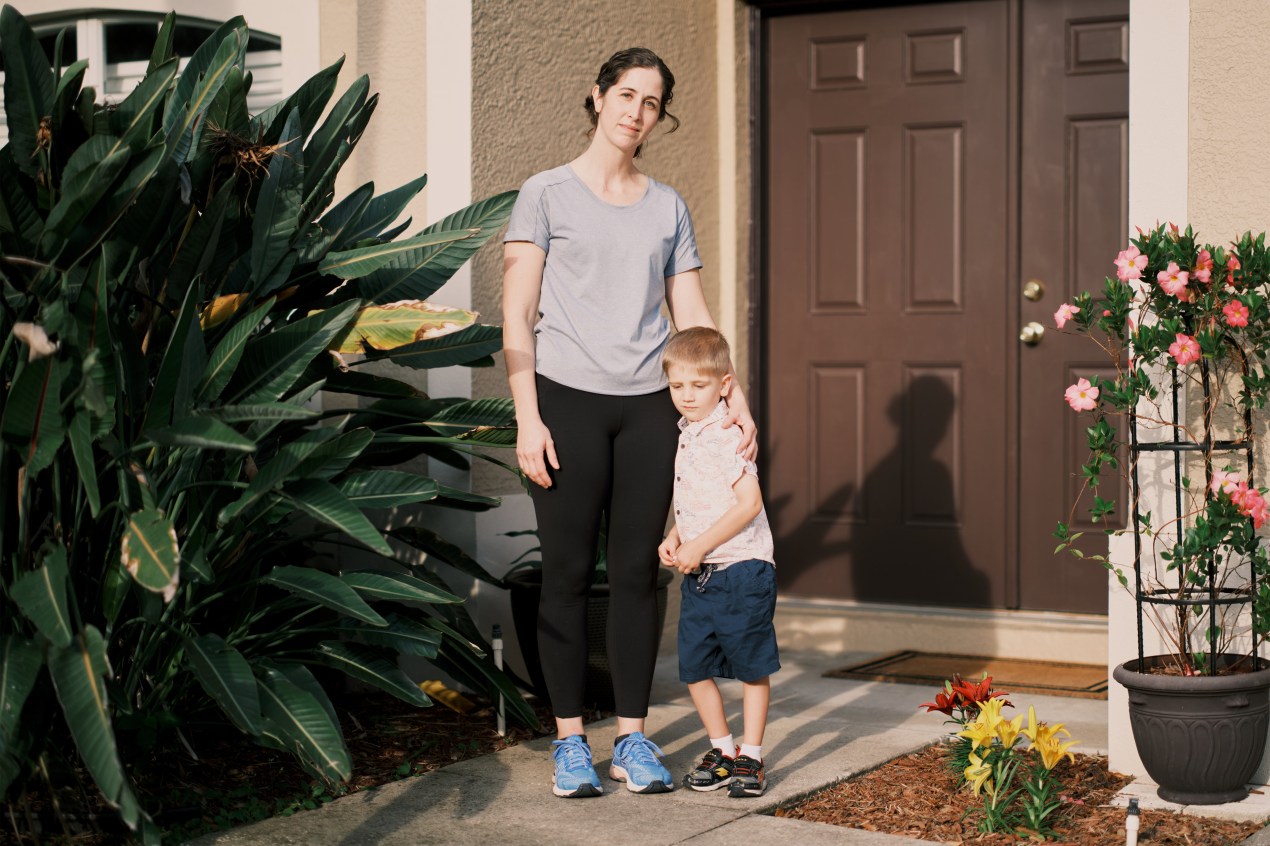Some hospitals sue patients who can’t afford to pay their medical bills. Such lawsuits don’t tend to bring in much money for the hospital but can really harm patients already experiencing financial hardships.
In this episode of “An Arm and a Leg,” Dan Weissmann goes toe-to-toe with Scott Purcell, CEO of ACA International, a trade association for the collection industry, on the effects these lawsuits have on patients.
With help from The Baltimore Banner and Scripps News, Weissmann pulls back the curtain on hospital bill lawsuits in three states — Maryland, Wisconsin, and New York — and discovers some good news for a change.
Dan Weissmann @danweissmann Host and producer of "An Arm and a Leg." Previously, Dan was a staff reporter for Marketplace and Chicago's WBEZ. His work also appears on All Things Considered, Marketplace, the BBC, 99 Percent Invisible, and Reveal, from the Center for Investigative Reporting.Credits
Emily Pisacreta Producer Adam Raymonda Audio wizard Ellen Weiss Editor Click to open the Transcript Transcript: ‘An Arm and a Leg’: When Hospitals Sue Patients (Part 2)Note: “An Arm and a Leg” uses speech-recognition software to generate transcripts, which may contain errors. Please use the transcript as a tool but check the corresponding audio before quoting the podcast.
Dan: Hey there – So, this is part two of a two-part story. If you missed part one, or just want a refresher, here’s three quick things:
First: Some hospitals – definitely not all – sue a LOT of patients over unpaid bills. Hundreds or even thousands every year.
Second: There’s very little money in it for these hospitals. When reporters and researchers add up the total amounts they’re suing for, it looks tiny compared to, say, their annual surplus. Or what they pay executives. Tiny.
Third: There’s data showing a LOT of the people being sued are … pretty hard up already.
That a lot of them would qualify for charity care under the hospitals’ own financial-assistance policies.
In fact, as we reported last time, a guy named Nick McLaughlin, who spent a decade working for a medical-bill collections agency… now runs a business telling hospitals they’d be better off – financially – writing these bills off through charity care or financial assistance programs.
And I should point out: Nick’s not a do-good crusader. He has started a business, to help hospitals do this. And he’s staked his family’s financial future on it.
Nick: I had a good but challenging conversation with my wife. And she said, hey, so is the reason we’re not doing this full time because we’re scared the money’s not gonna come in? And I said, well as the sole provider of a family of five that’s kind of a big deal. She said, yeah, I think we should do it.
Dan: And at the end of our last episode, I asked Nick: So, why would some hospitals make the decision to sue people, if there’s no money in it? What’s behind that decision:
Nick: It’s really, I would say, philosophically based.
Dan: So, in this episode, we’ll do two things: One, we’ll try to get a peek at that philosophy – inside the heads of the people who might hold it.
And TWO: We’re gonna share some hard data about what’s going on with these lawsuits in three states. We partnered with two awesome news organizations to get this data.
And I’m gonna tell you: we found what really looks like some good news.
And the whole inquiry really drove home ways we can help ourselves, and each other.
Here we go.
With Scripps News and the Baltimore Banner, this is An Arm and a Leg – a show about why health care costs so freaking much, and what we can may be do about it.
I’m Dan Weissmann. I’m a reporter, and I like a challenge. So our job on this show is to take one of the most enraging, terrifying, depressing parts of American life and bring you something entertaining, empowering, and useful.
So, let’s talk about that philosophy. You could call it a form of… not thinking too hard. Let’s start with a witness.
These days, Ruth Lande works for a nonprofit you may have heard of – RIP Medical Debt – to get hospital bills forgiven.
But WE talked with her because she spent more than 25 years working in hospital billing, most of it at Memorial Sloan-Kettering Cancer Center. And by the way, she loved it.
Ruth Landé: In general, I think it’s good if a job has three things. It’s for a good mission. Two, it should be hard. It should be complicated so it engages your brain every day. And third, it should be with really good colleagues. And I got to tell you, working revenue cycle satisfied all three of those for me.
Dan: And of course, during her quarter-century in the business, the question of whether or not to file lawsuits over hospital bills did come up.
When she got a promotion.
In her earlier role, she’d run one part of the billing department, where they never sued. Now she was taking over another part of the billing department, a bigger one, where sometimes they did.
She says her new colleagues were aware that in her earlier position, she’d taken a no-lawsuits approach.
Ruth Landé: There was an assumption, oh yeah, Ruth won’t allow that.
Dan: But, she told me, she didn’t want to be in conflict with her new colleagues from Day One.
Ruth Landé: And so I said, well, I’m not going to just ban it, but you know, bring me cases. If you believe that we should be suing a person, then just bring me the case so I can review it. And they never brought a case to me ever.
Dan: Never ever. She thinks those colleagues maybe hadn’t stopped to look at who they were suing.
Ruth Landé: When you really examine closely you see the harm. I They would have probably imagined that they’re only suing some really rich people sitting up in a mansion somewhere, not bothering to pay their bills.
You might imagine: It would be interesting to talk with someone who thinks this way – really talk with them, push them on their point of view.
And that did happen. Kind of.
It was honestly one of the most confusing conversations I’ve ever had. It was with this guy.
Scott Purcell: My name is Scott Purcell. I’m the CEO of ACA International.
Dan: That’s the industry association for folks in the bill-collection business. Scott was super-accommodating – got on Zoom with me within a day of my first email to him. So quickly that it wasn’t till we got on that I realized we hadn’t set a length.
Dan: How long do I actually have you for?
Scott Purcell: How long do you need us for?
Dan: Uh, I like to talk to people for a long time, but we start with a half an hour and maybe…
Scott Purcell: um, bum bum bum. I just need to change one meeting.
Dan: We talked for more than an hour.
The first half-hour was one kind of frustrating.
I’d describe our findings and findings from other people’s reports — for instance, how little money hospitals seem to gain from these lawsuits — and ask if he had data to help understand what we’re seeing, and he kept saying, effectively:
Hey, let’s not jump to policy conclusions. How would a new policy on debt collection affect a medical office with just three doctors?
Scott Purcell: And I would say that three person doctor office is different from one of the top 10 nonprofit health care system. Their economics are completely different. And yet we’re talking about policy positions. that impact both
Dan: And then, in retrospect I’ve figured out a spot where we really, really lost each other. I was talking about one observer’s take on why these lawsuits don’t bring in much money:
Dan: A lot of the people that end up as your defendants are effectively indigent. Um, you know, they don’t have a lot of income. They may not have W2 employment that you could garnish. They don’t have other assets you can take. So, the amount that you get is not, not what you might expect from looking at the number of cases and the number of judgments. So that was another…
Scott Purcell: If I could stop you there, I’d love to see that data. Do you know that it takes a lot of money to file a lawsuit? I can’t think. And so my lived experience, I cannot think of one instance where either the hospital or the collection agency or the attorney would choose to sue an indigent person because if they are going to have a low probability of being able to repay that that over time, why would you invest?
Dan: What I didn’t realize then, was: when I said some people were “effectively indigent,” Scott Purcell had latched onto the word “indigent” and had a very specific image in his mind, of absolute destitution. From that point forward, anything I would say about people being sued who were hard up, who qualified for charity care, who really couldn’t pay – was gonna run through this filter.
And: Any example I’d bring up of someone being sued who got put in an extremely tough position… was just gonna sound to him like a novel anecdote.
A half-hour in, I got pretty direct with Scott, so I asked:
Dan: How did this happen? How did it happen that we, like, got to the point where so many people are being sued over debts they can’t pay? What do you know about that?
And this is where things got really confusing to me. Because here’s how Scott responded:
Scott Purcell: Well, if you just sued somebody who can’t pay, they’re not going to pay you. So, they’re not out any money. So you made a bad business decision, but truly Dan, what is the harm they’re experiencing? The fact that they got sued and they can’t pay?
Dan: I didn’t see that coming – the idea that being sued could be “harmless”?. Here’s what I said:
My gosh. Well, I can tell you that, you know, people, by the time they’ve been sued, they’ve been getting tons of collections calls, their credit may have suffered, and they have a judgment against them that says like any money that shows up in their bank account can be seized or that, you know, the next time they get a job, their wages can be garnished. That’s pretty significant harm.
I described to Scott the story of Liz Jurado, a woman on Long Island who says she found out, years after the fact, that she had been sued over a bill relating to the birth of one of her kids. A bill she says she thought insurance had paid. Her husband was the main breadwinner, until he got laid off. Liz took a job working for DoorDash to support the family – her first W2 paycheck – and she says that’s how she found out about the lawsuit. Because once she starts the job, she starts getting letters, saying her wages are going to be garnished. And she’s like:
Liz Jurado: What is this? Where did it come from? How could they not tell me about it until now? I get a job and three months later, you’re coming after me. I mean, this is my family’s bread and butter. This is horrible.
Dan: I said to Scott: That seems bad, right?
Dan: So I’m, I’m, I’m trying to give you the opportunity to respond to that point that lots of people make that. If you get sued over a debt you can’t pay, there’s harm. That’s, that’s a lot of people’s positions, and I find it fairly persuasive. How do you respond to that?
Scott Purcell: You and I were using a hypothetical. You said somebody got sued who’s indigent. Has no money.
Dan: Do you think that doesn’t happen?
Scott Purcell: I don’t understand the business case as to why that would.
Dan: But, like, do you think it doesn’t happen because, like, do you think the reports that show that it happens a lot are wrong? I mean, I talked to a couple, a couple months ago who got sued over a debt. I mean, their story was like, they got hit with a bunch of medical problems.
I described to him the story of Casey and Ron Gasior, who we met in our last episode. The bills for those medical adventures threw their finances completely out of whack.
Casey: We would dig little bit out of our hole, and then we’d go right back down.
Dan: … until they were in danger of losing their house. They filed for chapter 13 bankruptcy – wrapping everything they owed into a five year payment plan. They’d just about made it through, when they got a letter from a law firm earlier this year: They were being sued over a medical bill, that had arrived just after their bankruptcy started. I was getting a little worked up.
Dan: So, these are not hypothetical, and these are not, like, you know, these stories are just entirely consistent with the data that, that gets collected. So, when you ask me, like, what’s the harm? I want to give you this opportunity to say, like, you sure that’s your position?
Scott Purcell: So, first of all, that was on a different, that was a different question. I made an assumption of that story that they were indigent now and would be indigent – I was saying, I don’t know why that decision got made if indeed that person, um, is indigent, why a particular, um, provider has whatever parameters they’ve set for their lawsuit program. I can’t speak to the business decisions they’re making. I can speak to, societally, what do we expect people to pay and not pay?
Dan: With the case of the couple in Wisconsin, if they couldn’t pay ever, if their chapter 13 hadn’t worked out, and they’d lost their house, and they’d lost their jobs, and they couldn’t pay ever, are you saying they wouldn’t be harmed?
Scott Purcell: I’m saying the answer lies in taking those stories to the table. And let’s take a look at what are the other policy changes that should be made in order to get better outcomes. So, in the situation you did outline, I am sure that individual actually went through emotional stress. But there’re safeguards throughout.
Dan: So you’re saying you view this as a kind of exceptional case and that generally there are, from what you know, guidelines and guardrails, as you say, to prevent this sort of thing from happening.
Scott Purcell: It’s the thing I don’t have data to answer it.
Dan: Yeah, it’s — I mean, I just need to say: It’s striking, um, that you asked — you’re, yeah, like: Where’s, where’s the harm?
Scott Purcell: I made an assumption of that story that they were indigent now and would be indigent–
Dan: Well, I guess I just don’t understand, I, I don’t really quite understand the difference. Can you explain the distinction between someone being indigent right now, being indigent forever, I don’t really get the distinction at all. And I don’t know in which case, in which case there is harm, in which case there isn’t in your view.
Scott Purcell: So, um, I wasn’t being flippant. I was taking a very extreme… um, I’m in D.C. I see homeless people now. So when I heard you say indigent, I’m thinking somebody who’s living under a bridge. They deserve to be treated with dignity and respect. I was thinking that level of indigency. You’re talking about, I think, the, the working class, and people beyond that. And up to the higher end scale is your question. And for that, my question or my answer is back to there are safeguards that should be occurring. And if those safeguards don’t occur, harm does happen. And we collectively need to look at why there are gaps in those safeguards.
Dan: So in retrospect – knowing how Scott Purcell took that word indigent – I’m a little less mystified. But the conversation still seems really… striking to me.
For one thing, there’s the idea — even if it’s not a conscious philosophy — that some people are beyond hope, so they’re beyond harm. So morally, it wouldn’t matter if, say, you sued them.
But the other thing that strikes me is the difficulty Scott Purcell had understanding – believing – that people being really harmed is something that happens at scale. That last thing he said: “There are safeguards that should be occurring, and IF those safeguards don’t occur, harm does happen.”
That word “IF” seems to be doing a lot of work there.
Beyond the mountains of data that folks have compiled – showing that people get sued who qualify for charity care, and that people who get sued over medical bills tend to live in neighborhoods where poverty is high – there’s the finding that’s practically a cliche:
About four out of ten Americans don’t have enough money on hand to cover a 400 hundred dollar emergency expense. Maybe I should have explained that to Scott Purcell.
But I just didn’t think I’d need to. He’s sitting atop a whole industry that NEEDS to know, basically, how much money people have. Since we talked, I’ve seen a report for folks in his industry – third-party collections – that goes into a lot of detail on that topic.
Of course, third-party collections agencies are for-profit businesses. And at least for some of them, lawsuits like these are part of the business.
So, I guess I’m starting to understand – maybe belatedly – how hard it is to get some people to reconsider business as usual. Is business as usual a philosophy?
But sometimes business as usual does change. In fact, I’m about to share some much more cheerful news with you. It’s what our partners found when we went looking for details on these hospital bill lawsuits in three states.
Because the big surprise was in what we DIDN’T find.
That’s coming right up.
This episode is produced in partnership with KFF Health News. That’s a nonprofit newsroom covering health care in America. Their incredible journalists win all kinds of awards every year. I’m so glad to get to work with them.
This investigation builds directly on reporting by KFF reporters like Jay Hancock, Noam Levey and Jordan Rau. Respect.
OK, so this whole inquiry — into why some hospitals sue so many patients who could just get charity care — started a couple of years ago.
That’s when I spotted what looked like a clue – in a big report done by National Nurses United. It looked at 145 thousand hospital lawsuits against patients in Maryland over a ten-year period.
And in addition to documenting how little money hospitals were getting from these suits — compared to the million-dollar salaries they paid a lot of executives —
This report also noted– just kind of by-the-way, on page 18 of a 68-page report – that a relatively small number of attorneys were filing most of these lawsuits.
Just five attorneys filed almost two-thirds of the cases.
And just one attorney filed more than 40,000 cases.
I was like, huh! Maybe that’s a clue.
It seems like hospitals don’t get a lot of benefit from these lawsuits. But maybe we’re looking at someone who does. We should find out more.
Starting with the names of those lawyers, which weren’t in the report.
And I was gonna want a big update on Maryland.
That report was part of a big advocacy campaign – which really worked.
In 2021, Maryland enacted a new law saying hospitals couldn’t sue anybody without checking to see if they qualified for free care.
Which in retrospect, may seem like an obvious requirement. Here’s Malcolm Heflin, one of the organizers who worked on the campaign.
Malcolm Heflin: It’s like reading the postscript in a Dickens novel almost. It’d be like, “Oh yeah. Hey, look, now we can’t chain children to factory machines.” Like what? Wait, what? That was legal before?
Dan: Anyway, if that report was the “before” picture, what would “after” look like? I was gonna need help. And I got some.
Ryan Little: my name is Ryan Little and I am the data editor at the Baltimore Banner.
Dan: The Banner is a new nonprofit daily newspaper – without the paper. Data reporting is a big specialty, and Ryan is the big specialist. Pulling a LOT of Maryland courts data was already on his to-do list.
Ryan Little: And so I said, maybe there’s a way that we can make a partnership happen. And then many months later, you’ve probably regretted that, but we’ve had a good time doing it. Anyways…
Dan: No way. Are you kidding me?
Ryan’s amazing. I am so lucky to get to work with him.
But I wanted to know about more than just Maryland. And I got lucky there too.
Maryland’s not the only state where advocates compiled a bunch of court data to push for change. You might remember Elisabeth Benjamin in New York from our last episode.
She’s the one who pointed out how little money is involved in these suits – for hospitals she has looked at.
Elisabeth Benjamin: They’re suing people for pennies. right. The average law suits maybe 1900 bucks. So they’re suing them for chump change, but that $1,900 is like life ruining for the patient.
Dan: She knew that because she had pulled more than 50 thousand hospital-bill lawsuits from across the state. She used that data in a series of reports that got new laws passed – like one banning wage garnishment to pay medical debts.
And she shared a giant spreadsheet with me, which included the names of attorneys in 40 thousand cases.
And guess what? Just three law firms handled the majority of those cases. So now we knew: This wasn’t just a Maryland thing.
But we were gonna want to look somewhere else too. Someplace where no new laws had been passed. Someplace that was still a “before” picture. Someplace like Wisconsin.
I’d been getting reports from a public-interest lawyer there named Bobby Peterson. He’d been publishing some data about lawsuits, but hadn’t gotten laws passed. And he also wasn’t able to share data. I was gonna need MORE help.
Rosie Cima: My name is Rosie Cima and I manage a data reporting team at Scripps News. I also report for them.
Dan: YES! More data help. Scripps News came aboard as a partner, and Rosie started looking for the data we’d need in Wisconsin.
And at this point, it may be getting clearer why it has taken us more than a year to bring this story to you. Let’s just recap for a second all the moving parts we’ve got in play here:
We’ve got Ryan, pulling cases in Maryland, Rosie doing the same in Wisconsin, and me with some New York cases.
We’re looking to see what the “after” picture looks like in Maryland and New York, and we’re looking at the role of a few lawyers.
And this is where I admit: that initial hypothesis? That the lawyers were driving these lawsuits, sweet-talking hospitals to drum up business?
It didn’t really pan out. As far as I can tell, after talking with a bunch of people and looking at a bunch of reports, it doesn’t seem to work that way.
A lot of the time, anyway, it seems like the lawyers are often freelancers. They get hired by the collection agencies.
Who get their marching orders from the hospital revenue office.
But I’m so glad we went looking, because of what we did find.
Or, you could say, what we didn’t.
In Maryland, Ryan spent months and months and months collecting hundreds of thousands of cases, then weeks and weeks crunching the numbers. And then…
Ryan Little: On Wednesday, September 6th, I sent this email. I find this hard to believe. But it may be that there were zero medical debt lawsuits filed by hospitals against individuals in 2022 and 2023.
Dan: He found it hard to believe – like, it must be wrong – so he went back to try to find his mistake. That took almost a week.
Ryan Little: On Monday, September 11th, I emailed, Hey Dan, news that hospital debt collection lawsuits had ended in Maryland was wrong. It looks like the Maryland Judiciary is somehow suppressing them in case search. Either intentionally or not, I’m rewriting the code to account for this.
Dan: He thought the Maryland court system was HIDING these cases. Not only did he rewrite the code, he went to the courthouse to go hunt for whatever was missing.
It took him another week. And then I got one more email.
Ryan Little: So on September 18th, I said, Maryland hospitals are dot, dot, dot. Basically not suing anyone for medical debt anymore.
Dan: Basically not suing anyone for medical debt this year. WOW. I mean, we had expected a significant drop– if only because Maryland had passed that 2021 law, which required hospitals to see if people were eligible for charity care before suing them.
But zero was a much bigger drop than we’d expected.
Next stop, New York. A few months ago, we looked at those three law firms – the ones that handled the majority of hospital-bill cases there.
And as far as we could tell, two of them were just not doing any work for hospitals at all anymore.
But OK, again: We’d expected an “after” picture in both these states. What about Wisconsin?
Well, for one thing, it turned out to be TOUGH.
Rosie Cima: When we took this on the first time, it definitely seemed like it’d be a lot easier than it ended up being.
Dan: You can pull some case data from the web, but there’s a problem: Once a case has been dismissed, it gets taken off that website after a few years.
Rosie Cima: So all the data that we had from before 2020 was missing some unknown number of cases
We can laugh about it now, but that sucked. We did find some guys who had data on older cases socked away. From them, we got the full caseloads for two lawyers we’d heard did a lot of medical-bill lawsuits.
Rosie Cima: We found more than 8000 cases in one year, um, for two lawyers,
Dan: That was 2019. Pre-pandemic.
Rosie Cima: And in 2022, There were fewer than 1400 for both of them.
Dan: In other words, these two lawyers were doing less than a quarter as much medical-bill business as they’d been doing three years earlier.
And Rosie pulled numbers year by year, client by client, which was super-revealing.
Because for both of them, many of their biggest clients – hospitals and medical practices for whom they had been filing hundreds of cases a year – weren’t filing any cases.
Which wasn’t totally conclusive. We knew these lawyers were getting less work…
Rosie Cima: The thing that we didn’t know was, like, whether, Hospital A had stopped suing, or whether they just stopped hiring this lawyer.
Dan: Right. So Rosie went back to the public data website to see whether those hospitals A, B, C and so on were suing. And for the most part, they weren’t — at least not like they used to.
Rosie Cima: Yeah, we now know that those cases weren’t going to a different lawyer. Right? They’re just not, they’re just not being filed.
Dan: Just not. Being filed. And it wasn’t just the hospitals that had been using these two lawyers that had fallen away. Other hospitals that had been suing tons of patients had cut way back.
From more than a thousand in 2019 to a few dozen, or less than a dozen. Or one. Or zero.
One hospital system sued more than 47 hundred people in 2019. In 2023 so far, they’ve sued one.
And remember, because older cases get wiped from the web, there’s some unknown number of cases from 2019 we aren’t seeing. The decline is probably bigger than what we see.
So, one thing to say is: We don’t know WHY this is happening. In any of these states. Our colleagues at the Baltimore Banner called every hospital in Maryland to ask about these changes, and got a bunch of no-comment. We emailed dozens of hospitals in Wisconsin and basically got the same answer.
So we’re left with some guessing – and here are some of our best guesses:
Those new laws in New York and Maryland didn’t outlaw lawsuits… but the Maryland law made them more difficult, and the New York laws made it harder to collect.
And the campaigns that led to those laws brought a LOT of negative attention to hospitals that filed a lot of lawsuits. So one way or another, it seems like a lot of hospitals decided it wasn’t worth it.
And in Wisconsin? Laws didn’t change, but the reports that the lawyer Bobby Peterson put out there did get some attention locally.
We know in Wisconsin, lawsuits halted altogether for a while when the pandemic started. Maybe hospitals noticed that they weren’t exactly losing a ton of money when that happened?
Here’s one last data point from Rosie. She looked closely at the cases she had for those two lawyers from 2019. The ones where the hospital was awarded a judgment.
Rosie Cima: We found that the majority of those awards were never fulfilled, like, I, I feel like that’s important, a judge said, yes, you defendant owe this case. company, the plaintiff, this much money and in a lot of cases, the plaintiff hasn’t paid out. And it’s been years.
Dan: Which I don’t think is evidence that “Wow, these folks were really good at dodging payment!” No, because in a lot of these old cases, the judge gave an OK to garnish these folks’ wages: To take money directly from their paycheck.
So if these debts haven’t been paid, years later – and remember, these are often amounts of a thousand dollars or less – it seems like these folks may be earning so little that garnishing their wages for years doesn’t get you much.
So, to start wrapping up: There’s a TON we don’t know. For one thing, there’s 47 other states we haven’t looked at. And we don’t know if hospitals in these three states will start suing again, when they think nobody’s looking.
But here’s something I do know: A surprising number of those other states have been passing new laws and regulations in the last couple years, to prevent hospitals from filing so many lawsuits against folks who qualify for charity care:
Illinois, Arizona, Colorado, Minnesota, Washington, Oregon. I’m probably missing some.
But here’s the single biggest thing I’m taking away from this whole adventure: A LOT more people qualify for charity care– free or discounted care from the hospital– than we think.
And we can help ourselves and each other, just by spreading the word.
I called Casey Gasior in Wisconsin a couple weeks ago. It wasn’t a great day for her.
Casey: Everybody in my house is sick and I just tested positive for covid. And now we’re going to lose work time.
Dan: Right.
Casey: I tell you, it never ends.
Dan: I was calling because I knew: Casey and her husband Ron have had more medical adventures this year. More knee trouble for him, emergency surgery for her, time away from work and lost income for both of them. And thousands of dollars of new medical bills.
I said to her: It seems like maybe you and Ron might qualify to have some of those bills forgiven through charity care.
Casey: I think my, my husband makes too much.
And I was like, well, maybe. But as we learned from Nick McLaughlin in our last episode, almost 60 percent of Americans qualify for charity care at a bunch of hospitals.
And the nonprofit Dollar For has created a database of the charity care policies of almost every hospital in the country – and they’ve built it into their website.
So you can type in a few details – where you were treated, how much you make – and it’ll tell you whether you’re likely to qualify for help.
Dan: So, I’m looking at their website right now.
And would it be okay with you to just kind of walk through kind of what they’re asking you, what they, um…
Casey: Yeah, sure.
Dan: Questions included: Where’d you get seen, and when?
Casey: Um, my surgery was July 24th.
Dan: Casey and I went line by line, filling out the form. I had her hunting for tax returns, and other documents
Casey: Hey, Ron. Can you send me a, um, a pay stub? Can you send me a picture of it? Like, now?
Dan: Okay. Alright, I’m going to add those up. There we go.
And yeah, so Dollar For thinks that you would qualify,
Casey: Wow. That surprises me.
Dan: This is good.
Casey: This is really…
Dan: Yeah. I’m really glad that we took this step.
Casey: Yeah, me too, because I was kind of, I didn’t know where to go and like, it, it seems so weird asking for charity.
Dan: But Casey was ready to take the next step.
Casey: Now this application that I’m filling out now do I have to do one for myself and one for Ron.
Dan: Yes. Yeah.
Casey: Okay, I’m going to work on this
Dan: Okay. Fantastic.
And this is a thing that we can do for ourselves, and each other. Spread the word: The majority of people qualify for at least some charity care – at least partially wiping out your bill – at a LOT of hospitals.
The Dollar For website is set up to tell you if you’re likely to qualify, and to help you apply. They’ve also got actual human beings on staff to help if you get stuck.
Their website is Dollar For – that’s Dollar F-O-R dot org. Dollar F-O-R dot org.
And that is our story. We never got all the way to the bottom of the question of WHY these bulk lawsuits happened – or why they seem to have stopped in some places – but we did get a peek into the process.
And we learned some things that are heartening – a lot fewer lawsuits in these three states!
I’ve learned a lot more, along the way – there’ll be follow-ups.
This has been a HUGE project for our little outfit. We got a ton of help from our partners, and we put a TON of resources into it: Travel to Wisconsin and Michigan, MONTHS of phone calls, 1600 bucks to get court records.
We’ve been able to do that because you’ve been supporting us– giving us the resources to do the job. And this is the absolute best time to pitch in:
Every dollar you give is matched. A few generous Arm and a Leg listeners have put up more than 10 thousand dollars in matching funds ON TOP of what the Institute for Nonprofit News does through their NewsMatch program – and I want to max it out.
The place to go is Arm and a Leg Show, dot org, slash support. And there’s a link in the show notes – pretty much anywhere you’re listening to this.
We’ll be back next week with a quick little coda to this story.
Meanwhile, thank you so much for helping us make this show. I’m gonna give that address one more time: Arm and a Leg show dot com, slash support.
I’ll catch you next week.
Till then, take care of yourself.
This episode of An Arm and a Leg was produced by me, Dan Weissmann, with Emily Pisacreta and Bella Czakowski.
In partnership with Scripps News, thanks to Rosie Chima, Amber Strong, Claire Malloy, Jacqueline Baylon and Zach Toombs and the Baltimore Banner, thanks to Ryan Little, Meredith Cohn, Brenna Smith and Kimi Yoshino and the McGraw Center for Business Journalism at the Craig Newmark Graduate School of Journalism at the City University of New York, with thanks to Jane Sasseen.
Our work on this story is supported by the Fund for Investigative Journalism, and edited by Ellen Weiss.
Big thanks also to Jared Walker, Bobby Peterson, Luke Messac, Jeff Bloom, Emily Stuart, Berneta Hayes, Matt Szaflarski, Amanda Dunkler, and Marceline White! Plus Barry and Jo from Court Data Techologies, in Wisconsin.
Gabrielle Healy is An Arm and a Leg’s managing editor for audience – she edits the First Aid Kit newsletter.
Sarah Ballema is our Operations Manager. Bea Bosco is our Consulting Director of Operations.
An Arm and a Leg is produced in partnership with KFF Health News.
That’s a national newsroom producing in-depth journalism about health care in America, and a core program at KFF — an independent source of health policy research, polling, and journalism.
You can learn more about KFF Health News at arm and a leg show dot com, slash KFF.
Zach Dyer is senior audio producer at KFF Health News. He is an editorial liaison to this show.
Thanks to the INSTITUTE FOR NONPROFIT NEWS for serving as our fiscal sponsor, allowing us to accept tax-exempt donations. You can learn more about INN at I-N-N dot org.
And thanks to everybody who supports this show financially.
If you haven’t yet, we’d love for you to pitch in to join us. Again, the place for that is arm and a leg show dot com, slash support.
And now, time for one of my favorite parts: Shouting out some of the folks who have made donations since our last episode. Thanks this time to…
[DAN READS NAMES]
Thank you so much!
“An Arm and a Leg” is a co-production of KFF Health News and Public Road Productions.
This episode was produced in partnership with Scripps News, The Baltimore Banner, and the McGraw Center for Business Journalism at the Craig Newmark Graduate School of Journalism at the City University of New York.
Work by “An Arm and a Leg” on this article is supported by the Fund for Investigative Journalism.
To keep in touch with “An Arm and a Leg,” subscribe to the newsletter. You can also follow the show on Facebook and X, formerly known as Twitter. And if you’ve got stories to tell about the health care system, the producers would love to hear from you.
To hear all KFF Health News podcasts, click here.
And subscribe to “An Arm and a Leg” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
from Health Industry Archives - KFF Health News